Question 1: Which separation techniques will you apply for the separation of the following?
(a) Sodium chloride from its solution in water.
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride.
(c) Small pieces of metal in the engine oil of a car.
(d) Different pigments from an extract of flower petals.
(e) Butter from curd.
(f) Oil from water.
(g) Tea leaves from tea.
(h) Iron pins from sand.
(i) Wheat grains from husk.
(j) Fine mud particles suspended in water.
Answer:
(a) Evaporation
(b) Sublimation
(c) Filtration
(d) Chromatography
(e) Centrifugation
(f) Separating funnel
(g) Filtration
(h) Magnetic separation
(i) Winnowing/sedimentation
(j) Decantation and filtration
Question 2: Write the steps you would use for making tea. Use the words, solution, solvent, solute, dissolve, soluble, insoluble, filtrate, and residue.
Answer:
1. Take a cup of water in a container as a solvent and heat it.
2. Add sugar in it which is solute. Heat it till all sugar dissolves.
3. You get a solution of water and sugar.
4. Sugar is soluble in water completely.
5. Add half a tea-spoon of tea-leaves, it is insoluble in water.
6. Boil the content, add milk which is also soluble in water, boil again.
7. Filter the tea with the help of a strainer, the tea collected in the cup is filtrate, and the tea leaves collected on the strainer are the residue.
Question 3: Pragya tested the solubility of three different substances at different temperatures and collected the data as given below (results are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).
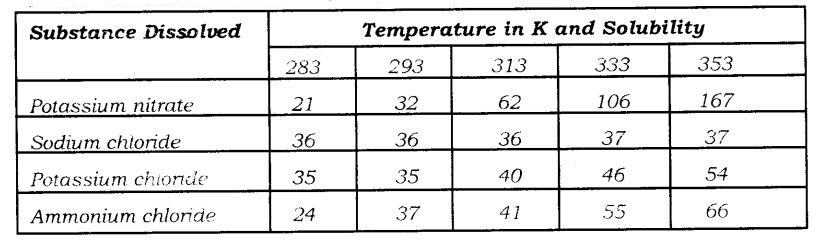

Question 4: Explain the following giving examples:
(a) Saturated solution
(b) Pure substance
(c) Colloid
(d) Suspension
Answer:
(a) Saturated solution: In a given solvent when no more solute can dissolve further at a given temperature is called a saturated solution.
(b) Pure substance: A pure substance consists of a single type of particles. E.g., gold, silver.
(c) Colloid: A colloid is a solution in which the size of solute particles is bigger than that of a true solution. These particles cannot be seen with our naked eyes, they are stable, e.g., ink, blood.
(d) Suspension: It is a heterogeneous mixture in which the solute particles are big enough to settle down, e.g., chalk-water, paints, etc.
Question 5: Classify each of the following as a homogeneous or heterogeneous mixture:
soda water, wood, air, soil, vinegar, filtered tea.
Answer:
Homogeneous: Soda water, vinegar, filtered tea.
Heterogeneous: Wood, air, soil.
Question 6: How would you confirm that a colorless liquid given to you is pure water?
Answer: By finding the boiling point of a given colorless liquid. If the liquid boils at 100°C at atmospheric pressure, then it is pure water. This is because pure substances have a fixed melting and boiling point.
Question 7: Which of the following materials fall in the category of a "pure substance"?
(a) Ice (b) Milk (c) Iron
(d) Hydrochloric acid (e) Calcium oxide (f) Mercury
(g) Back (h) Wood (i) Air.
Answer: Pure substances are: Ice, iron, hydrochloric acid, calcium oxide, and mercury.
Question 8: Identify the solutions among the following mixtures.
(a) Soil (b) Sea water
(c) Air (d) Coal
(e) Soda water.
Answer: Solutions are: Sea water, soda water, and air.