Question 1: Convert the following temperatures to the Celsius scale.
(a) 293 K
(b) 470 K
Answer:
(a) 293 K into °C = 293 – 273 = 20°C
(b) 470 K into °C = 470 – 273 = 197°C
Question 2: Convert the following temperatures to the Kelvin scale.
(a) 25°C
(b) 373°C
Answer:
(a) 25°C into K = 25 + 273 = 298 K
(b) 373°C into K = 373 + 273 = 646 K
Question 3: Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
Answer:
(a) Naphthalene balls disappear with time without leaving any solid because naphthalene balls sublime and directly change into vapor state without leaving any solid.
(b) We can get the smell of perfume sitting several metres away because perfume contains volatile solvents that diffuse faster and can reach people sitting several meters away.
Question 4: Arrange the following substances in increasing order of forces of attraction between the particles—water, sugar, oxygen.
Answer: Oxygen —> water —> sugar.
Question 5: What is the physical state of water at—
(a) 25°C
(b) 0°C
(c) 100°C
Answer:
(a) 25°C is liquid
(b) 0°C is solid or liquid
(c) 100°C is liquid and gas
Question 6: Give two reasons to justify
(a) water at room temperature is a liquid.
(b) an iron almirah is a solid at room temperature.
Answer:
(a) Water at room temperature is a liquid because its freezing point is 0°C and boiling point is 100°C.
(b) An iron almirah is a solid at room temperature because the melting point of iron is higher than room temperature.
Question 7: Why is ice at 273 K more effective in cooling than water at the same temperature?
Answer: Ice at 273 K will absorb heat energy or latent heat from the medium to overcome the fusion to become water. Hence the cooling effect of ice is more than water at the same temperature because water does not absorb this extra heat from the medium.
Question 8: What produces more severe burns, boiling water or steam?
Answer: Steam at 100°C will produce more severe burns as extra heat is hidden in it called latent heat whereas boiling water does not have this hidden heat.
Question 9: Name A, B, C, D, E, and F in the following diagram showing a change in its state:
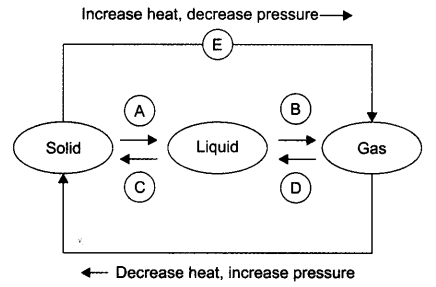
Answer:
A —> Liquefaction/melting/fusion
B —> Vaporization/evaporation
C —> Condensation
D —> Solidification
E —> Sublimation
F —> Sublimation